The Burning Of Fossil Fuels And Its Effects
Last updated: 27 Dec 2023
What are Fossil Fuels?
Fossil fuels are made from decomposing remains of plants and animals. Hence, they can be found buried in the Earth’s crust. These fuels contain carbon and hydrogen, which can be burned for energy. Some examples of fossil fuels will include coal, natural gas and crude oil.
The Burning of Fossil Fuels
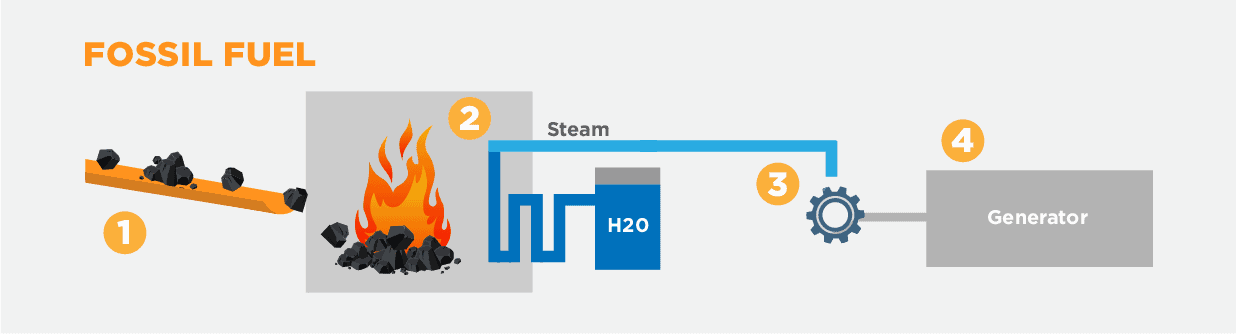
- Fossil fuel (such as coal, natural gas or oil) is burned in a furnace, turning chemical energy into heat.
- Water is piped through the furnace, heating up into pressured steam.
- The steam is funnelled toward a turbine, making it turn.
- The turbine drives a generator, which turns the original heat energy into electricity.
Effects of the Burning of Fossil Fuels on the Environment
-
Global warming
The burning of any fossil fuel will produce carbon dioxide, which contributes to the greenhouse effect warming the Earth. When the greenhouse gases in the environment increases, the temperature of the atmosphere is expected to increase, further driving the current global warming crisis. The damage doesn’t stop there. We see the results of increased global temperature in the destruction of ecosystems such as the Great Barrier Reef, drastic changes in the world’s weather patterns, the melting of glaciers and polar ice as well as the rise in sea levels resulting in the frequent cases of flooding.
-
Air pollution
Besides carbon dioxide, the burning of fossil fuels will lead to the emission of carbon monoxide, nitrogen oxides, sulphur oxides, and hydrocarbons. These pollutants will then cause various forms of air pollution. The combination of sulphur dioxide, nitrogen oxide and nitrogen dioxide can produce acid rain. In addition, carbon monoxide and nitrogen oxide come together to form tropospheric ozone, the major constituent of smog. Consequently, smog will lead to a list of respiratory illnesses.
-
Ocean acidification
When carbon dioxide produced from the burning of fossil fuels goes into our ocean waters, it reacts with seawater to form carbonic acid. Since the start of the Industrial Revolution when we start burning coals, the ocean’s acidity has increased by 30%. When the acidity goes up, calcium carbonate—a substance used by oysters, lobsters, and countless other marine organisms to form shells—goes down. This results in slow growth rates, weaken shells and could imperil entire food chains. Ocean acidification does not only harm commercially important shellfish, such as lobster, crabs, and mussels, but also other species in marine food webs. These could very likely cause a ripple effect and affect other fishes, birds, and mammals.
